Author Archives: Roberto Castelnovo
Are your Data Management Policies supporting the GXP compliance?
Understanding the criticality of setting Master Data and Policies
Your Data lifecycle extends across departments and its governance requires good practices to be followed. Understanding the criticality’s of setting common Master Data and policies is very important and extended educational efforts should be considered at corporate level.
When implementing digital solutions for quality processes, GxP activities, the data management policies should apply to any computer software’s involved, all along the data lifecycle, data workflows and related processes, with particular attention to Read More...
6 Factors for a successful Implementation of a Laboratory Information Management System (LIMS)
Have you implemented a Laboratory Information Management Solution without really benefiting from all its capabilities?
Unfortunately, it is frequent to discover that the Laboratory Information Management Solution implemented in a site is not providing all the resources and insights that the laboratory and its stakeholders would benefit from.
Too many factors influence the success of a system implementation, irrespective of the LIMS providers, the type of lab or the size. Too often the project gets delayed. Too often phase 2 never Read More...
How to be sure that your Data Integrity project has fully covered your eData LifeCycle?
The integrity of your Data along their lifecycle
This is not another data integrity article, yet this is about demonstrating to auditors that you do have it all right and under control. So we’d like to highlight the importance of assessing the complete life cycle of your data and managing it for complete compliance to data integrity requirements.
We’ve all attended the thousand trainings, webinars and presentation about Data Integrity recommendations and guidelines. We know by heart the ALCOA definition and Read More...
Data Integrity, Company Integrity: a Management Commitment
Thousands of documents and articles have been written about Data Integrity. You probably have already attended several presentations and webinars introducing the concept, repeating incessantly the list of data integrity guidelines published by the regulatory bodies and showing you frightening summaries of warning letters.
Data integrity is not a new trend
You know that data integrity is not a new concept. You know of multiple examples not even related to the pharma industries that had a strong impact in the society. Read More...
Review by Exception: the ultimate Laboratory 4.0 objective
In a previous article, we described the evolution of the laboratories along the four industrial revolutions, in which the last one gets the machines, devices, sensors, and people connecting, communicating and driving effective production. The principles of the fourth revolution are interconnection, transparency of the information, technical assistance, decentralized decisions.
Technicians and analysts have the ability to connect and communicate with their analytical instruments, devices, and sensors. Capture of the data is automatic, controlled and compliant towards data integrity requirements. Read More...
Practical session for your laboratory digital transformation project
SETTING THE STAGE FOR YOUR PAPERLESS LABORATORY TRANSFORMATION
[Monday, 8th April 2019 – 14:00-18:00 CET] Gran Hotel Dino, Baveno, Lake Maggiore, Italy
The Paperless Lab Academy 2019 is now up and running and registrations are open. We’re glad to inform you that we are offering, on the pre-congress day, a special training composed of multiple practical sessions for setting the stage for your paperless laboratory transformation.
Roberto Castelnovo will speak about how to design the most adequate digital transformation strategy for your Read More...
The Laboratory of the Industry 4.0
Organizing an event like the Paperless Lab Academy requires strong project management skills, execution at its best and a lot of energy. However, the very best part of it is the outstanding opportunities to engage with so many active members of the lab informatics industry and its final users.
While discussing the content of their presentation or workshop for the #PLA2019 agenda, we definitely enjoy discovering people analysis on their today pains and their vision of the lab of the Read More...
Setting the stage for a paperless laboratory
The intelligent analytical laboratory is becoming reality: an automated laboratory where data, methods and processes are seamlessly shared between software applications and analytical instruments. The ultimate goal for a digitalized laboratory is to leverage the power of the data and provide useful information, business intelligence for decision makers sitting at any step of the data life cycle. Fact is that rarely the laboratory reaches the point to feel completely automated and free of paper processes. For a reason or Read More...
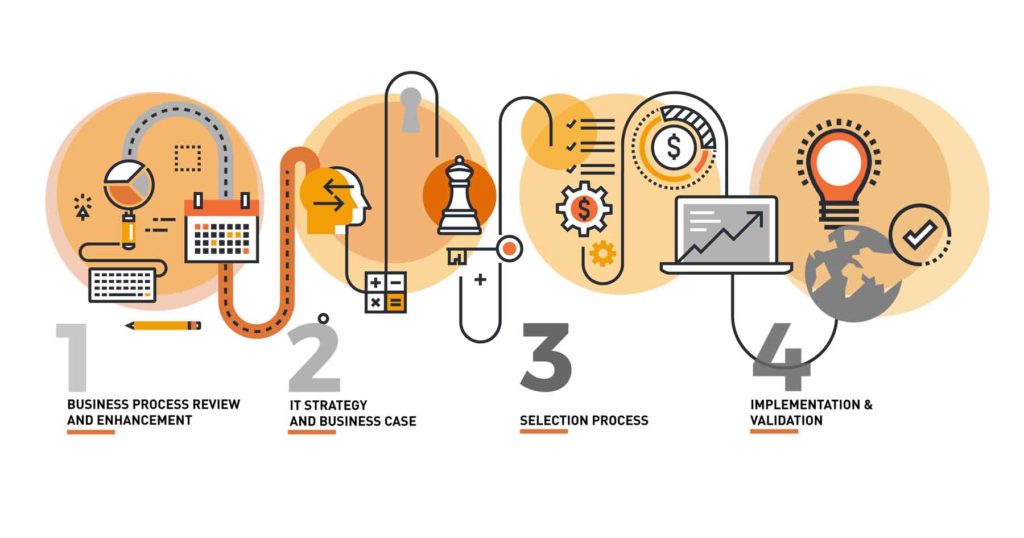
Selection Process II: 5 steps in the selection of a LIMS
As mentioned in our previous article about How to select a LIMS, it is highly recommended traveling the path to reduce the emotional part of the purchase and select the right tool with the end in mind. These are the 5 steps to minimize the risk of failure in selecting a digital tool for your laboratory processes.
5 steps to select your data management solution
In this article, we start with the assumption that you have walked your way to define Read More...
Selection process – How to select a LIMS
At one point of a digital transformation project for the laboratory processes, the moment comes to identify the best fit-to-purpose system to manage the laboratory scientific data. As mentioned in previous articles, this step should be by far one of the last in your paperless project. All previous steps will provide you commitment from the team members and stakeholders, will streamline your processes and will ensure a successful implementation.
Readiness: key differentiator for the process of selecting a laboratory data Read More...